Nick Hunt: The Hidden Pfizer Report That Shows Up to 40% More Heart Conditions in the Vaccinated
The report in question is Pfizer’s report C4591021 ‘Interim Report 5’ dated March 12th 2024.
One-time or recurring donations can be made through Ko-Fi:
For six months, the MHRA and other national regulators have been sitting on a Pfizer report about Covid vaccine safety. Worryingly, the abstract which I have just found online doesn’t look good at all:
the vaccinated cohort have at least 23-40% higher risk of some heart-related conditions; and
the risk is higher than in Pfizer’s previous report (i.e., it is increasing over time since vaccination).
The report in question is Pfizer’s report C4591021 ‘Interim Report 5’ dated March 12th 2024. It is a Post Authorisation Safety Study (PASS) of Pfizer’s Covid vaccine. In summary, national regulators routinely require pharmaceutical manufacturers to conduct PASS studies as a condition of authorisation of most new medicines. The regulators provide data to the manufacturer covering millions of patients registered in national healthcare systems. The manufacturer then conducts analysis to determine whether the medicine has increased the risk of specified health conditions.
I have previously written a couple of articles about Covid vaccine PASS studies. First, in October 2023, to raise awareness of the studies and the fact that most of them were not being published. Second, in January 2024, to report that I had obtained copies of PASS studies by Pfizer, Moderna and AstraZeneca via a Freedom of Information request to the MHRA. In the second article I picked out three health conditions (arrhythmia, heart failure and acute coronary artery disease) from Pfizer’s ‘Interim Report 4’ where there was a higher incident rate in the vaccinated cohort.
Knowing that Pfizer had completed its ‘Interim Report 5’ in March 2024, in April I submitted FOI 24/075 to MHRA asking for a copy. MHRA applied a Section 22 Exemption: “information intended for future publication.” This seemed very odd given that it had sent me previous ones only three months before. However, helpfully, it stated that it “will be published in the fourth quarter of 2024”.
So in late August, I submitted another FOI (24/475) to check that this was still MHRA’s intention. Imagine my surprise when it backtracked: “We cannot confirm whether the Pfizer C4591021 Interim Study Report 5 prior to December 31st 2024 is still due to be published. We have contacted the company, who have informed us that the final report is due for submission at the end of 2024 and plans for publication will be decided at this point.” I read that as: “We’re worried about the results in Interim Report 5, so we’ve decided to wait for Pfizer’s Final Report before deciding if and when to publish either of them.”
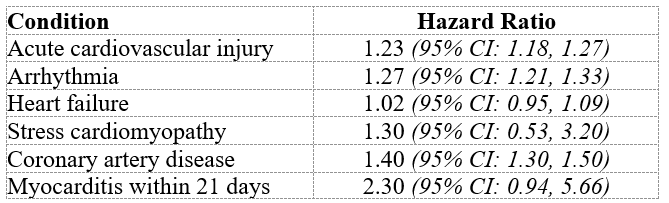
Imagine my further surprise when I just found an abstract of Pfizer’s ‘Interim Report 5’ online. As I said at the start, it doesn’t look good. Here are the first six conditions mentioned in the abstract:
Now, a Hazard Ratio of 1.23 means that the condition is 23% more likely in the vaccinated cohort, and “CI” mean confidence interval, i.e., we can be 95% confident that the ‘true’ number lies between the following two numbers. So those data are extremely worrying. This is the manufacturer bearing out the numerous anecdotal reports of increasing heart issues since 2020 as well as various independent research reports.
Worse, those data are worse than the corresponding figures in Pfizer’s previous ‘Interim Report 4’. In other words, the risk appears to be increasing over time since Covid vaccination.
And by the way, none of the above can be attributed to Covid itself: the exposure to Covid will be broadly the same in both the vaccinated and unvaccinated cohorts, which comprise millions of individual patients.
That said, there are potential confounders. The abstract suggests two:
that for any condition, the seriousness might vary within and between the cohorts; and
‘healthy vaccinee’ bias – the argument that vaccinated individuals are more likely to seek medical attention.
But that’s one reason why we need to see the whole Pfizer report – to see the whole dataset, results and argumentation which lead to Pfizer’s explanation about confounding.
Even more importantly, we need to see the whole report because the Hazard Ratio will vary by age: younger people are normally much less prone to heart-related conditions than older people. Imagine how surprised I will be if the Hazard Ratios in the full report for younger age groups are even worse than those in the abstract (which are averages across all age groups). Is MHRA sitting on information which actually confirms the many siren warnings that it was reckless for MHRA to authorise, and JCVI to recommend, Covid vaccination of younger people who were at extremely low risk from Covid when it was known at the time that the Covid vaccines didn’t stop transmission and there were no long term safety data?
In summary, if, as I suspect, MHRA is worried by the results in Pfizer’s ‘Interim Report 5’ then no wonder it is sitting on it.
One final thought. The Covid Inquiry Module 4 (Vaccine & Therapeutics) oral hearings are scheduled for January 14th-25th 2025. It would be a travesty if Pfizer’s ‘Interim Report 5’ and ‘Final Report’ were withheld from the inquiry. Perhaps one of the core participants or their legal representatives will request copies or question MHRA about the data at the oral hearings.
Until Nick retired a few years ago, he was a Senior Civil Servant in the Ministry of Defence responsible for the safety and effectiveness of ammunition used by the Armed Forces. He is co-author of the Perseus Group report on U.K. medicines regulator the MHRA.
Related articles: